New mRNA vaccine for malaria uses cancer immunotherapy adjuvant to improve efficacy

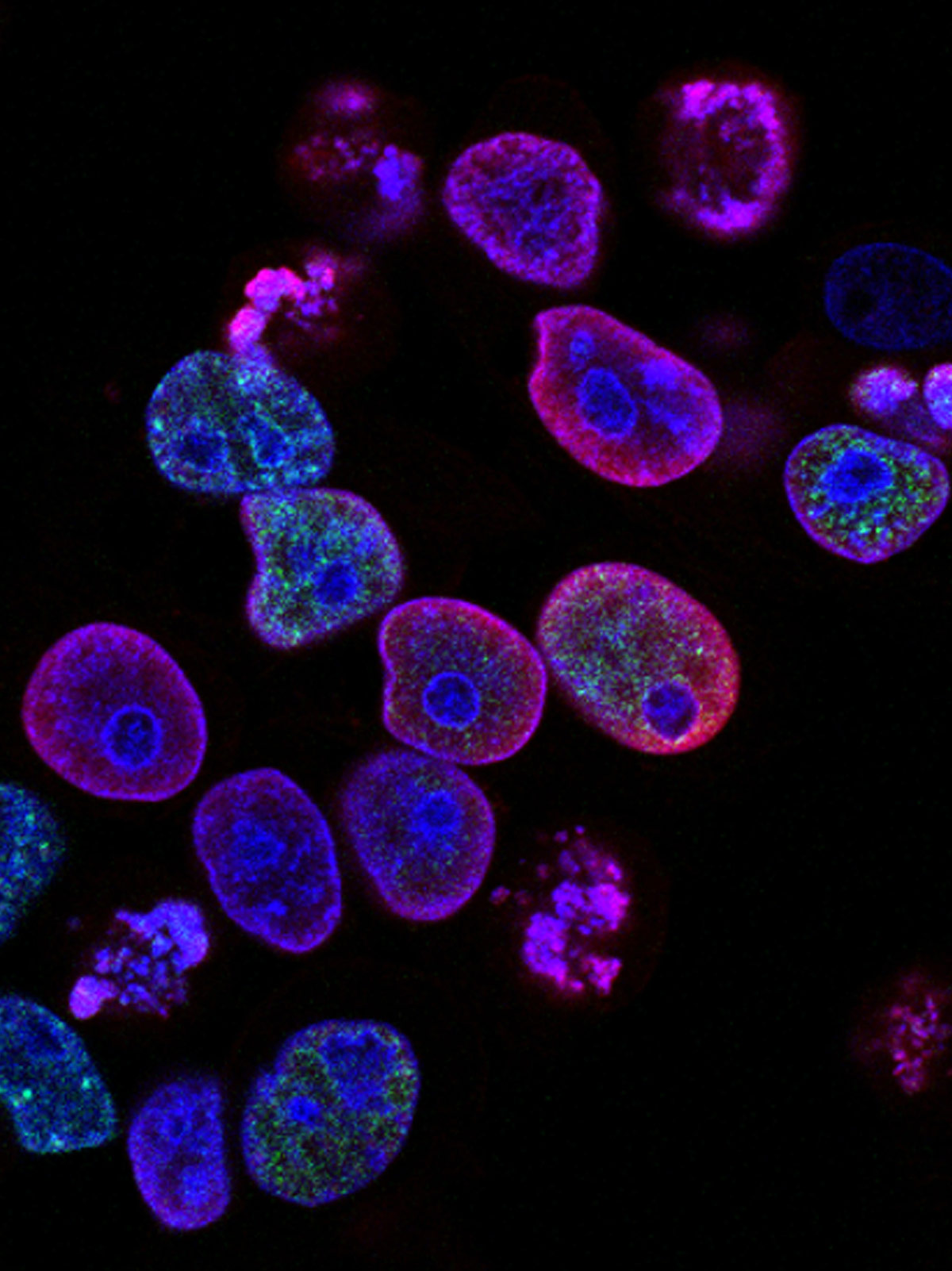
In an article published July 20 in Nature Immunotherapy, researchers from New Zealand’s Ferrier Research Institute, the Malaghan Institute of Medical Research and Australia’s Peter Doherty Institute for Infection and Immunity showed their novel malaria vaccine generates an immune response in mouse models, and was able to prevent infection with Plasmodium berghei, a form of the parasite.
It was also effective in animals that had already been exposed to malaria, a key differentiator from other vaccines.
“A lot of malaria vaccines undergoing trials have worked really well in animal models or when they’re given to people who haven’t had malaria before, but they don’t work well when given to people living in malaria-endemic regions.” co-author Lauren Holz, Ph.D., a researcher at the Peter Doherty Institute, said in a press release. “In contrast, our vaccine is still capable of generating protective liver-specific immune cells and providing protection even when the animal models have been pre-exposed to the disease.”
There’s only one malaria vaccine widely available right now—Mosquirix, developed by GlaxoSmithKline, which was officially rolled out to the world in 2022. It generates antibodies to specific proteins found on the surface of malaria sporozoites, an immature form of the parasite.
Though it’s unquestionably a feat, Mosquirix’s efficacy rate leaves something to be desired: On average, it prevents malaria infection 35% of the time, though it staves off severe disease 50% of the time during the first year after administration. A similar vaccine from the University of Oxford was approved in Ghana in April; this one has a higher efficacy rate, at 77%.
Meanwhile, several companies and research groups are working on mRNA vaccines for malaria. That includes COVID-19 vaccinemaker BioNTech, which announced in December 2022 that it had launched a clinical trial of its malaria vaccine, BNT165. The same month, a research group out of George Washington University and the University of Pennsylvania—co-led by Drew Weissman, M.D., Ph.D., one of the pioneers of mRNA technology—showed that its mRNA malaria vaccine series could prevent both infection and transmission of the parasite by targeting different stages of the parasite’s life cycle.
Like other mRNA vaccines for malaria, the Ferrier, Malaghan and Peter Doherty institutes’ vaccine encodes the entire malaria protein rather than select antigens, the way protein-based vaccines such as Mosquirix do. But unlike other mRNA vaccines, like the ones used for COVID-19, their shot is designed to upregulate memory T-cells in the liver, an approach made possible through the use of an adjuvant the Ferrier and Malagahn institutes had developed to boost the immune system’s response to cancer.
“Unlike the COVID-19 vaccine that works by neutralizing antibodies, our unique approach relies on T-cells which play a critical role in immunity,” co-author Mitch Ganley, Ph.D. said in the release. “Specifically, a type of T-cell called a tissue-resident memory T-cell, that halts malaria infection in the liver to completely stop the spread of infection.”
After confirming that the vaccine generated an immune response in mice, the researchers tested it in challenge experiments against Plasmodium berghei sporozoites in mice that hadn’t previously been exposed to malaria.
Fourteen of the 15 mice in the group that received the adjuvant-boosted malaria vaccine were protected from developing malaria; none of the 15 mice in the group without the adjuvant saw the same, nor did the 12 in the control group. Protection increased with a second dose of the vaccine.
The researchers then conducted another set of challenge experiments, this time adding a group of mice that had been infected with malaria previously. In this case, the vaccine with the adjuvant protected seven of the 10 uninfected mice, and all nine of the mice that had had an infection.
“Together, these results indicate that the suppressive effects of blood-stage preexposure seen for attenuated sporozoite vaccines do not extend to immunization by adjuvanted mRNA vaccines, potentially a major advantage for translation into the field,” the researchers concluded in their paper.
Next, the researchers plan to test their vaccines in humanized mouse models as they move toward translational studies, Holz relayed to Fierce Biotech Research in an email. They anticipate entering clinical trials in several years.